Archives
br Author s contributions br Conflict of interest br Acknowl
Author's contributions
Conflict of interest
Acknowledgements
This work was supported in part by a grant from Department of Biotechnology (DBT), Government of India (Sanction Order No.: BT/563/NE/U-Excel/2016). MC, PDG and SKM acknowledge the Ministry of Human Resource and Development (MHRD), Government of India for their research scholarship.
Introduction
Transporter proteins are integral membrane proteins critical to the uptake, distribution, and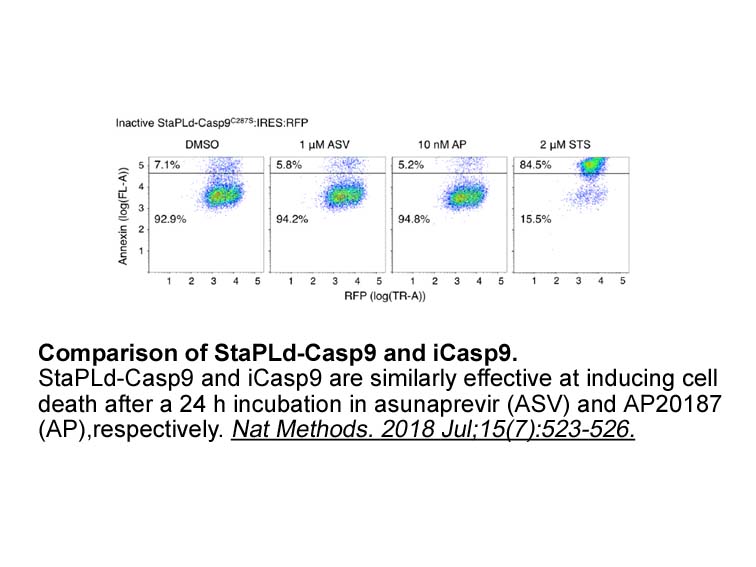 excretion of endogenous compounds and xenobiotics such as nutrients, hormones, bile acids, peptides, lipids, sugars, and drugs (Cesar-Razquin et al., 2015). They can be broadly categorized into two superfamilies: the SoLute Carrier (SLC) transporters comprising over 400 integral membrane proteins subdivided into 50+ families and the ATP-Binding Cassette (ABC) superfamily, consisting of 7 families (ABCA through ABCG). Membrane transporters are inherently hydrophobic and large transmembrane domains span the cellular membrane (Harris & Booth, 2012; Harris, Findlay, Simms, Liu, & Booth, 2014). Despite their hydrophobicity, transporters have dynamic structures, and can adopt conformations that are not readily captured via X-ray crystallography. Therefore, specific proteoforms identified biochemically and computationally are often relied upon for mechanistic insight into the function of these proteins (Schlessinger, Khuri, Giacomini, & Sali, 2013; Schlessinger, Yee, Sali, & Giacomini, 2013; Shaikh et al., 2013). Post-translational modifications (PTMs) are regulators of these structural events and are critical for the transporters' structure, function, and regulation within the confines of the lipid environment. The hydrophilic loops and termini that face the intracellular milieu may be accessible for these modifications if the exposed amino SC 79 side chains are solvent accessible, providing a mechanism in which the chemical nature of an amino acid can be altered (Walsh, Garneau-Tsodikova, & Gatto, 2005).
While there are 400+ types of PTMs that have been identified to date, the most common variants that are known to play a role (or, those that have been actively investigated) in the regulation of transporters include phosphorylation, glycosylation, and ubiquitination. Biochemically, these modifications diversify the nature of the amino acid peptide-backbone or side chain through the addition of small chemical groups (e.g. phosphates), lipids (e.g. palmitic acid), carbohydrates (e.g. mannose), small proteins (e.g. SUMO), among other entities (Duan & Walther, 2015; Hunter, 2007; Korkuc & Walther, 2017; Prabakaran, Lippens, Steen, & Gunawardena, 2012; Schlessinger, Khuri, et al., 2013; Schlessinger, Yee, et al., 2013; Shaikh et al., 2013; Walsh et al., 2005). Most, if not all, eukaryotic proteins undergo PTMs, and the likelihood for a given modification to occur is driven by the amino acid sequence, the structural and chemical constraints of the protein surface, and the availability of the necessary protein machinery and precursors to facilitate the modification (Fig. 1) (Duan & Walther, 2015; Korkuc & Walther, 2017). For transporters, this likelihood is further complicated by lipid-protein interactions driven by the buried transmembrane domains, which are obligatory to the functional expression of the protein. As such, solvent accessible residues located within the hydrophilic loops and termini of the membrane transporter often contain canonical consensus motifs (Table 1) that serve as recognition sites for not just the PTM, but also for the required adaptor proteins and enzymes needed to facilitate the modification (Amanchy et al., 2007; Dietrich & Ungermann, 2004; Guan & Fierke, 2011; Knorre, Kudryashova, & Godovikova, 2009; Rodriguez, Dargemont, & Hay, 2001; Walsh et al., 2005). While common consensus motifs are conserved across the proteome, there also are non-canonical recognition motifs that have been identified under physiologically relevant contexts (Kaneko, Joshi, Feller, & Li, 2012).
excretion of endogenous compounds and xenobiotics such as nutrients, hormones, bile acids, peptides, lipids, sugars, and drugs (Cesar-Razquin et al., 2015). They can be broadly categorized into two superfamilies: the SoLute Carrier (SLC) transporters comprising over 400 integral membrane proteins subdivided into 50+ families and the ATP-Binding Cassette (ABC) superfamily, consisting of 7 families (ABCA through ABCG). Membrane transporters are inherently hydrophobic and large transmembrane domains span the cellular membrane (Harris & Booth, 2012; Harris, Findlay, Simms, Liu, & Booth, 2014). Despite their hydrophobicity, transporters have dynamic structures, and can adopt conformations that are not readily captured via X-ray crystallography. Therefore, specific proteoforms identified biochemically and computationally are often relied upon for mechanistic insight into the function of these proteins (Schlessinger, Khuri, Giacomini, & Sali, 2013; Schlessinger, Yee, Sali, & Giacomini, 2013; Shaikh et al., 2013). Post-translational modifications (PTMs) are regulators of these structural events and are critical for the transporters' structure, function, and regulation within the confines of the lipid environment. The hydrophilic loops and termini that face the intracellular milieu may be accessible for these modifications if the exposed amino SC 79 side chains are solvent accessible, providing a mechanism in which the chemical nature of an amino acid can be altered (Walsh, Garneau-Tsodikova, & Gatto, 2005).
While there are 400+ types of PTMs that have been identified to date, the most common variants that are known to play a role (or, those that have been actively investigated) in the regulation of transporters include phosphorylation, glycosylation, and ubiquitination. Biochemically, these modifications diversify the nature of the amino acid peptide-backbone or side chain through the addition of small chemical groups (e.g. phosphates), lipids (e.g. palmitic acid), carbohydrates (e.g. mannose), small proteins (e.g. SUMO), among other entities (Duan & Walther, 2015; Hunter, 2007; Korkuc & Walther, 2017; Prabakaran, Lippens, Steen, & Gunawardena, 2012; Schlessinger, Khuri, et al., 2013; Schlessinger, Yee, et al., 2013; Shaikh et al., 2013; Walsh et al., 2005). Most, if not all, eukaryotic proteins undergo PTMs, and the likelihood for a given modification to occur is driven by the amino acid sequence, the structural and chemical constraints of the protein surface, and the availability of the necessary protein machinery and precursors to facilitate the modification (Fig. 1) (Duan & Walther, 2015; Korkuc & Walther, 2017). For transporters, this likelihood is further complicated by lipid-protein interactions driven by the buried transmembrane domains, which are obligatory to the functional expression of the protein. As such, solvent accessible residues located within the hydrophilic loops and termini of the membrane transporter often contain canonical consensus motifs (Table 1) that serve as recognition sites for not just the PTM, but also for the required adaptor proteins and enzymes needed to facilitate the modification (Amanchy et al., 2007; Dietrich & Ungermann, 2004; Guan & Fierke, 2011; Knorre, Kudryashova, & Godovikova, 2009; Rodriguez, Dargemont, & Hay, 2001; Walsh et al., 2005). While common consensus motifs are conserved across the proteome, there also are non-canonical recognition motifs that have been identified under physiologically relevant contexts (Kaneko, Joshi, Feller, & Li, 2012).